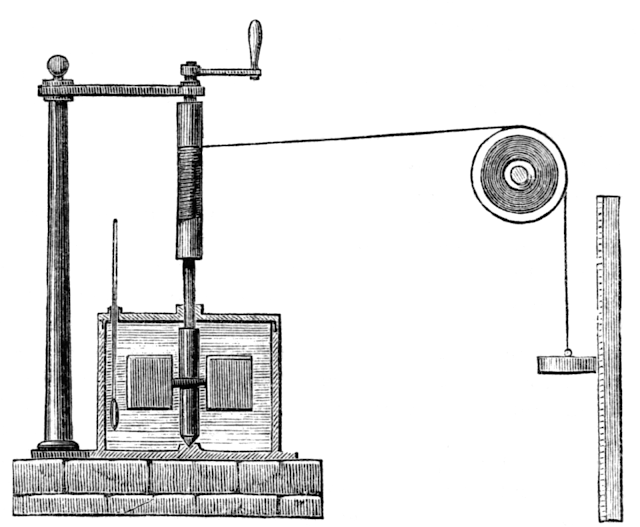
 |
| James Prescott Joule's apparatus. Credit: Harper's New Monthly Magazine, No. 231, August 1869 |
By Andrew Bennett
For many years, people did not understand the nature of temperature. In an experiment that combined measurements of motion (and the energy associated with it) and temperature, James Prescott Joule established that the energy associated with motion and position is the same as the energy associated with heat.
If you would like to receive alerts when I update this blog and my YouTube channel, please subscribe. Thank you!
Richard Feynman had a great way of describing how all this works at the atomic level. Check out an interview where he describes atoms "jiggling" and how we perceive it as temperature.
How Do We Apply the Results of Joule's Experiment?
This establishes some key ideas for our investigations of processes involving compressing, expanding, heating, and cooling of gases.
At the end of this video, we work through a conservation of energy problem that has energy entering or leaving the system both as work and as heat.
At the end of this video, we work through a conservation of energy problem that has energy entering or leaving the system both as work and as heat.
If viewing via email, click here to watch the video.
More Heat and Energy Videos
For more information on the Law of Conservation of Energy, check out this playlist. For more information about thermodynamics, check out this playlist.
If you would like to receive alerts when I update this blog and my YouTube channel, please subscribe. Thank you!
Comments
Post a Comment